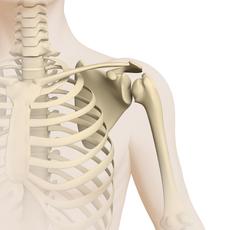
Osteosarcoma (Brief information)
An Osteosarcoma is a rare malignant tumour of the bones. This text provides information about the characteristics of this disease, its frequencies, causes, symptoms, diagnosis, treatment, and prognosis.
Author: Maria Yiallouros, Dr. med. habil. Gesche Tallen, erstellt am: 2009/02/12, Editor: Maria Yiallouros, Reviewer: Prof. Dr. med. Stefan Bielack, Dr. med. Dorothee Carrle, Dr. Stefanie Hecker-Nolting, English Translation: PD Dr. med. Gesche Tallen, Last modification: 2020/02/04 doi:10.1591/poh.patinfo.osteosarkom.kurz.20101215
Table of contents
General information on the disease
Osteosarcomas are rare malignant bone tumours. They are solid tumours arising from malignantly transformed bone-forming cells. Since osteosarcomas develop directly from bone tissue, they are also called primary bone tumours in order to distinguish them from cancers of other body parts that have spread to the bones (bone metastases). Most osteosarcomas grow and spread very quickly. Without an appropriate treatment, the disease is fatal.
Incidence
Osteosarcoma is the most frequent malignant bone tumour in childhood and adolescence. According to the German Childhood Cancer Registry (Mainz, Germany), about 2 to 3 of 1,000,000 (in total approximately 40) children and adolescents under 15 years of age are diagnosed with osteosarcoma in Germany per year, which accounts for about 2.3 % of all paediatric malignancies in this age group.
The frequency of this type of cancer, however, peaks during the pubertal growth spurt in the second decade of life. Hence, osteosarcomas mostly occur in adolescents between 15 and 19 years of age, thereby accounting for more than 5 % of malignant diseases in this age range. In girls, the incidence of osteosarcoma peaks at 14 years, in boys at 16 years of age. Overall, boys are more frequently affected by the disease than girls.
Localisation and spread
Most osteosarcomas arise in a long bone of an extremity (arm, leg), usually in those areas that are close to the joints (metaphyses). More than 50 % of all osteosarcomas are located around the knee.
The tumour sometimes affects bone and/or bone marrow only, but it frequently also spreads into adjacent tissue, such as connective, fat, muscle or nerve tissue. In about 10 to 20 % of children and adolescents with osteosarcoma tumour spread (metastases) is detectable at diagnosis.
It has to be assumed for all other patients, too, that tumour cells have already spread elsewhere via the blood or lymphatic stream and thus formed tiny metastases in other organs. These so-called micrometastases may not be detected at diagnosis due to their small size.
Osteosarcomas primarily metastasizes to the lungs (about 70 %), less frequently to bones and other organs. Sometimes, metastases develop both in lungs and bones at the same time. Very rarely (in less than 5 % of the patients), tumours are found in various different bones from the very beginning (multifocal disease).
Histologic characteristics and tumour types
One major characteristic of osteosarcoma is that the tumour cells – in contrast to healthy bone-forming cells – produce immature bone tissue (osteoid). That means that they only form the unmineralised portion of the bone matrix. This feature alone allows to distinguish osteosarcoma from other bone tumours.
Apart from that, however, the histologic characteristics of osteosarcoma (i.e. the way the tumour cells look under a microscope) are diverse as is the biological behaviour of these tumours. Most osteosarcomas in childhood and adolescence are highly malignant, since they grow rapidly and spread fast. Only a few subtypes of osteosarcoma may be considered to be of low or medium grade of malignancy.
The World Health Organization (WHO) differentiates between the following subtypes of osteosarcoma based on their typical histologic features (WHO classification of bone tumours):
- conventional osteosarcoma (high grade of malignancy)
- telangiectatic osteosarcoma (high grade of malignancy)
- small-cell osteosarcoma (high grade of malignancy)
- low-grade central osteosarcoma (low grade of malignancy)
- secondary osteosarcoma (usually high grade of malignancy)
- parosteal osteosarcoma (usually low grade of malignancy)
- periosteal osteosarcoma (medium grade of malignancy)
- high-grade surface osteosarcoma (high grade of malignancy)
Conventional osteosarcomas are the most frequent. They account for about 80 to 90 % of all osteosarcomas and are subclassified according to the WHO classification. All other subtypes are rather rare (accounting for less than 5 % each). The grade of malignancy has a major impact on treatment planning.
Causes
The causes of osteosarcoma are only partially understood. Genetic, growth-related as well as various other factors are currently being considered to all contribute to the risk of developing osteosarcoma.
The latter include ionising radiation as used, for example, in radiotherapy, as well as certain cellular poisons (cytotoxins), such as certain anticancer agents. Radiation and cytotoxins are capable of causing genetic instability in bone-forming cells, thereby contributing to initiate osteosarcoma development.
Also, children and adolescents with certain hereditary diseases, such as bilateral retinoblastoma, Li-Fraumeni syndrome or Bloom syndrome, have an increased risk to develop osteosarcoma, as do children with chronic bone diseases like Paget disease. For most patients (90 %), however, no particular cause can be found.
Symptoms
The most frequent symptoms of osteosarcoma are pain in a bone or joint and/or a swelling over a bone or bony part of the body.
Pain may be intermittant, activity-dependent and – with increasing tumour growth – accompanied by a lump at the tumour site. The swelling may be reddened and warm and may sometimes lead to impaired mobility, which initially may be mistaken for the result of a sports injury or bone inflammation.
Sometimes, even a minor accident may cause a fracture at this site (pathological fracture). In about 5 % of patients, bone fracture represents the first symptom of the disease. The symptoms described are caused by the tumour growth within the tender bone, which is very sensitive to pain, and adjacent soft tissue.
Patients with advanced disease may also complain of general symptoms such as fever, weight loss and/or fatigue. For some patients, only a few weeks, for others up to several months may pass between first symptoms and the diagnosis of osteosarcoma.
Good to know: Not all children and adolescents presenting with the complaints described above suffer from osteosarcoma or any other malignant bone tumour. However, every type of musculoskeletal pain in a child or a teenager should be taken seriously and be dealt with by an experienced paediatrician in order to appropriately rule out an underlying cancer.
Diagnosis
If the paediatrician thinks that the young patient’s history and physical exam are suspicious of a malignant bone tumour, the child should immediately be referred to a hospital with a childhood cancer program (paediatric oncology unit), where further diagnostics can be initiated and performed by childhood cancer professionals.
Very close collaboration between various specialists (such as paediatric oncologists, paediatric surgeons, paediatric radiologists, to name a few) is required both to find out whether the patient really suffers from a malignant bone tumour and, if so, to determine the tumour type and the extension of the disease. Knowing these details is absolutely essential for optimal treatment planning and prognosis.
Imaging tests and tissue removal (biopsy)
Based on their typical radiological features, most malignant bone tumours can already be diagnosed on an x-ray. Additional imaging procedures such as magnetic resonance imaging (MRI) and computed tomography (CT) subsequently help to define the exact tumour site and size as well as its demarcation with regard to adjacent tissue (such as muscles, tendons or joints). Nearby metastases (called skip metastases), too, are easily detectable by these methods.
For imaging of affected soft tissue and bone marrow, MRI is superior to CT. Apart from plain x-rays, it is therefore considered as the gold standard in radiological diagnosis of osteosarcoma. For final confirmation of the diagnosis, however, tumour tissue is required to be removed (biopsy) and subsequently viewed under a microscope by experts (histologic examination).
Diagnostic procedures to assess tumour spread
In order to assess potential tumour spread into other organs (metastasis), usually a chest x-ray as well as a chest-CT and a bone scintigraphy are performed. Various research studies currently examine the efficacy and feasibility of additional imaging techniques for osteosarcoma diagnostics, such as positron emission tomography (PET).
Tests for treatment preparation
Before treatment begins, further tests are needed in order to assess the condition of different organs. Therefore, the doctors will recommend an electrocardiography (ECG) as well as an ultrasound of the heart (echocardiography), a hearing test (audiometry), special diagnostics for determining kidney and lung functions as well as various blood tests.
These tests will be repeated regularly during the course of treatment. If any of these follow-up results change compaired to those obtained by the initial tests, therapy can be accordingly adjusted.
Treatment planning
After the diagnosis has been confirmed, therapy is planned. In order to design a highly individual, risk-adapted treatment regimen for the patient, certain individual factors influencing the patient’s chance of recovery (prognosis) – called risk factors or prognostic factors – are being considered during treatment planning (risk-adapted treatment strategy).
Important prognostic factors are the type, the localisation, size and spread of the tumour, which are assessed before treatment starts. In the course of treatment, additional prognostic factors, such as the extent of tumour removal (incomplete versus complete) and the response of the disease to chemotherapy, are of major impact on how treatment is going to be continued. Taking into account all these factors aims at achieving the best outcome possible for each patient.
Treatment
Treatment of children and adolescents with osteosarcoma basically consists of surgery (local therapy) and chemotherapy. Only patients with the rare low-grade subtypes may benefit from surgery alone. Radiotherapy plays an inferior role. It is only considered when complete tumour removal is not possible. Overall, treatment takes about nine to twelve months.
Treatment consists of the following phases:
Chemotherapy prior to surgery
For most patients, therapy is initiated by a ten-week phase of chemotherapy (called preoperative, neoadjuvant or induction chemotherapy). The goal of this chemotherapy is to shrink the tumour and potential spread (metastases) in order to optimise the conditions for subsequent surgical tumour removal, thereby contributing to safety and efficacy of surgery. Also, chemotherapy helps eliminate micrometastases and prevent further tumour spread.
In order to eliminate as many tumour cells as possible, patients receive a combination of different chemotherapeutic agents that have proven to be effective in the treatment of osteosarcoma. These agents include methotrexate, adriamycin (doxorubicin) and cisplatin (MAP).
Chemotherapy is given as several courses over several days each, during which the patient needs to be inpatient. Between courses, patients can go home as outpatients. Only in case of severe side-effects, readmission is required.
Surgery
Following chemotherapy, patients undergo surgery with the goal of complete surgical tumour removal. Also, metastases need to be removed to increase the chances of cure. Thanks to major technical progress regarding limb-saving surgical procedures, tumour removal does usually not require amputation any more.
Following surgery, a pathologist examines the tumour tissue to find out how the disease has responded to the preceding chemotherapy. This response is assessed by measuring the amount of the tumour cells that are still alive. An amount of less than 10 % is considered as good response, which is achieved for about 50 % of all osteosarcoma patients. For patients whose tumour and/or metastases could not be completely removed, radiotherapy might be another treatment option.
Chemotherapy after surgery
After surgery, chemotherapy with methotrexate, adriamycin and cisplatin (MAP) is continued for 18 weeks (postoperative chemotherapy).
Treatment of recurrent disease
Like the primary disease, recurrent osteosarcoma (relapse) requires complete removal of the tumour in order to achieve cure.
Patients with single lung metastases, in particular if these occur later than two to three years after primary diagnosis, may benefit from surgery alone. Other patients with relapsed osteosarcoma will also need another round of chemotherapy, for example with carboplatin, etoposide and ifosfamide. In palliative settings, radiotherapy may be an option. Overall, the prognosis for patients with relapsed osteosarcoma is not favourable.
Therapy optimising trials / registries
In the large paediatriac treatment centres, children and adolescents with osteosarcoma receive therapy according to standardised treatment plans (protocols). These protocols are designed by experts and aim at steadily improving the patients’ survival rates while also reducing the risk of therapy-related late effects.
Therapy according to such treatment protocols is usually carried out within "therapy optimising trials". The term refers to a certain form of controlled clinical trial studying the efficacy of current treatment concepts for sick patients in order to improve them based on the current scientific knowledge.
In Germany, patients with osteosarcoma had the option to be enrolled in the therapy optimising trial EURAMOS 1 until June 2011. The study was conducted by the Cooperative Osteosarcoma Study Group COSS of the German Society for Paediatric Oncology and Haematology (GPOH) in collaboration with other renowned groups. Numerous children’s hospitals and treatment centres all over Germany and other European as well as North American countries participated.
Since the EURAMOS trial has been closed for patient admission, children and adolescents with osteosarcoma are being registered in the so-called COSS-Registry until the subsequent trial is ready to be opened. The German headquarters of trial and registry are located at the Children’s Hospital of the Olgahospital in Stuttgart, Germany. Principal investigator is Professor Dr. med. Stefan Bielack.
Peculiarity of a clinical registry: Patients who cannot participate in any study, for example because none is available or open for them at that time, or since they do not meet the required inclusion criteria, respectively, may be included in a so-called registry. Such a registry pools scarce data in order to help with the planning of appropriate future clinical trials. To ensure optimal treatment for patients not registered in a study, experts from assigned trial panels usually provide recommendations and advice to the local caregiver team.
Prognosis
The chances of survival (prognosis) for children and teenagers with an osteosarcoma depend on various factors. In particular, the subtype and the site of the tumour, its extent at diagnosis, response to preoperative chemotherapy as well as the extent of surgical tumour removal are of major prognostic importance.
During the last four decades, prognosis of children and teenagers with osteosarcoma has continuously been increased by major improvements of diagnostics and treatment in the framework of therapy optimising trials.
By combining different treatment methods and particularly by introducing intensive, standardised combination chemotherapy regimens, survival rates of more than 60 % can be achieved today. Favourable prognosis usually requires complete tumour removal and good response to the chemotherapy that precedes surgery.
Patients with non-metastasised osteosarcoma of the arm or leg have the most favourable prognosis – cure rates may reach up to 70 %, depending on the response to chemotherapy. Patients with good response (that means less than 10 % of living tumour cells after chemotherapy) have a much better prognosis than patients with poor response. The latter have a high risk of recurrent disease (relapse); the probability of disease-free survival is currently less than 50 %.
Patients with osteosarcoma of the trunk or with large tumours have an overall unfavourable prognosis compared to those with a tumour of the limbs or with small osteosarcoma, respectively. In case of metastases, their localisation, thus operability, is a major prognostic factor. Patients with single, removable lung metastases have a better chance of survival than patients with bone metastases or multifocal disease.
Note: The survival rates mentioned in the text above are statistical values. Therefore, they only provide information on the total cohort of patients with osteosarcoma. They do not predict individual outcomes.
In the context of cancer, the term "cure" should rather be referred to as "free of cancer", because current treatment regimens may help to destroy the tumour, but they are also frequently associated with numerous late-effects. Early detection and appropriate management of these long-term secondary effects typically require intensive rehabilitation and thorough long-term follow-up care, although a patient may have been "cured" from the cancer.
References 
- Kaatsch P, Grabow D, Spix C: German Childhood Cancer Registry - Jahresbericht / Annual Report 2016 (1980-2015). Institut für Medizinische Biometrie, Epidemiologie und Informatik (IMBEI), Universitätsmedizin der Johannes Gutenberg-Universität Mainz 2016 [URI: www.kinderkrebsregister.de]
- Marina N, Smeland S, Bielack S, Bernstein M, Jovic G, Krailo M, Hook J, Arndt C, van den Berg H, Brennan B, Brichard B, Brown K, Butterfass-Bahloul T, Calaminus G, Daldrup-Link H, Erksson M, Gebhardt M, Gelderblom H, Gerss J, Goldsby R, Goorin A, Gorlick R, Grier H, Hale J, Hall K, Hardes J, Hawkins D, Helmke K, Hogendoorn P, Isakoff M, Janeway K, Jürgens H, Kager L, Kühne T, Lau C, Leavey P, Lessnick S, Mascarenhas L, Meyers P, Mottl H, Nathrath M, Papai Z, Randall R, Reichardt P, Renard M, Safwat A, Schwartz C, Stevens M, Strauss S, Teot L, Werner M, Sydes M, Whelan J: Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial . Lancet Oncol 2016, 17: 1396 [PMID: 27569442]
- Whelan JS, Bielack SS, Marina N, Smeland S, Jovic G, Hook JM, Krailo M, Anninga J, Butterfass-Bahloul T, Böhling T, Calaminus G, Capra M, Deffenbaugh C, Dhooge C, Eriksson M, Flanagan AM, Gelderblom H, Goorin A, Gorlick R, Gosheger G, Grimer RJ, Hall KS, Helmke K, Hogendoorn PC, Jundt G, Kager L, Kuehne T, Lau CC, Letson GD, Meyer J, Meyers PA, Morris C, Mottl H, Nadel H, Nagarajan R, Randall RL, Schomberg P, Schwarz R, Teot LA, Sydes MR, Bernstein M, EURAMOS collaborators: EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2015, 26: 407 [PMID: 25421877]
- Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Pápai Z, Meyer J, Nadel H, Randall RL, Deffenbaugh C, Nagarajan R, Brennan B, Letson GD, Teot LA, Goorin A, Baumhoer D, Kager L, Werner M, Lau CC, Sundby Hall K, Gelderblom H, Meyers P, Gorlick R, Windhager R, Helmke K, Eriksson M, Hoogerbrugge PM, Schomberg P, Tunn PU, Kühne T, Jürgens H, van den Berg H, Böhling T, Picton S, Renard M, Reichardt P, Gerss J, Butterfass-Bahloul T, Morris C, Hogendoorn PC, Seddon B, Calaminus G, Michelagnoli M, Dhooge C, Sydes MR, Bernstein M, EURAMOS-1 investigators: Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. Journal of clinical oncology 2015, 33: 2279 [PMID: 26033801]
- Kager L, Bielack S: [Chemotherapeutic concepts for bone sarcomas]. Der Unfallchirurg 2014, 117: 517 [PMID: 24903502]
- Bielack S: Osteosarkome. Leitlinie der Gesellschaft für Pädiatrische Onkologie und Hämatologie AWMF online, 2010 [URI: www.awmf.org]
- Bielack S, Carrle D: Diagnostik und multimodales Therapiekonzept des Osteosarkoms. ärztliches journal reise & medizin onkologie, Otto Hoffmanns Verlag GmbH 3/2007, S: 34
- Zoubek A, Windhager R, Bielack S: Osteosarkome. in: Gadner H, Gaedicke G, Niemeyer CH, Ritter J (Hrsg.): Pädiatrische Hämatologie und Onkologie Springer-Verlag 2006, 882 [ISBN: 3540037020]
- Bielack S, Machatschek J, Flege S, Jürgens H: Delaying surgery with chemotherapy for osteosarcoma of the extremities. Expert Opin Pharmacother 2004, 5: 1243 [PMID: 15163270]
- Graf N: Osteosarkome. in: Gutjahr P (Hrsg.): Krebs bei Kindern und Jugendlichen Deutscher Ärzte-Verlag, 5. Aufl. 2004, 473 [ISBN: 3769104285]
- Lion TH, Kovar H: Tumorgenetik, in Gutjahr P: Krebs bei Kindern und Jugendlichen. Deutscher Ärzte-Verlag Köln 5. Aufl. 2004, 10 [ISBN: 3769104285]
- Bielack S, Kempf-Bielack B, Delling G, Exner G, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K: Prognostic factors in high-grade osteosarcoma of the extremities or trunk. J Clin Oncol 2002, 20: 776 [PMID: 11821461]
- Bielack S, Flege S, Kempf-Bielack B: Behandlungskonzept des Osteosarkoms. Onkologe 2000, 6: 747 [DOI: 10.1007/s007610070064]
- Bielack S, Kempf-Bielack B, Schwenzer D, Birkfellner T, Delling G, Ewerbeck V, Exner G, Fuchs N, Göbel U, Graf N, Heise U, Helmke K, von Hochstetter A, Jürgens H, Maas R, Munchow N, Salzer-Kuntschik M, Treuner J, Veltmann U, Werner M, Winkelmann W, Zoubek A, Kotz R: Neoadjuvant therapy for localized osteosarcoma of extremities. Results from the Cooperative osteosarcoma study group COSS of 925 patients. Klin Pädiatr 1999, 211: 260 [PMID: 10472560]
 Brief information on Osteosarcoma (PDF) (200KB)
Brief information on Osteosarcoma (PDF) (200KB)
Author: Maria Yiallouros, Dr. med. habil. Gesche Tallen
24/08/2017