Low-Grade Glioma (LGG) - Brief Information
Low-grade gliomas are rare tumours of the central nervous system (CNS). This text provides information about the characteristics of this disease, its frequencies, causes, symptoms, diagnosis, treatment, and prognosis.
Author: Maria Yiallouros; Dr. med. habil. Gesche Tallen, erstellt am: 2007/04/20, Editor: Maria Yiallouros, Reviewer: Dr. med. Astrid Gnekow; Dr. Daniela Kandels, Last modification: 2021/08/27 doi:10.1591/poh.patinfo.ng.kurz.1.20070627
Table of contents
General information on the disease
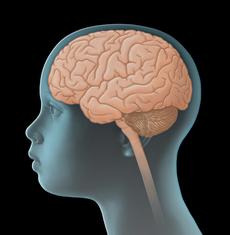
Low-grade gliomas are tumours of the central nervous system (CNS). They are solid tumours arising from malignantly transformed cells of the brain or spinal cord. Since they develop directly from CNS cells, they are also called primary CNS tumours in order to distinguish them from cancers of other body parts that have spread to the CNS (metastasis). Low-grade gliomas can be found in all parts of the nervous system, most of them, however, are situated in the cerebellum and the central regions of the cerebrum, such as the optic pathway (optic pathway gliomas) and the hypothalamic-pituitary axis.
The growth pattern of low-grade gliomas is unpredictable. In most patients, these tumours are localised and grow slowly. Some patients even experience phases of no tumour growth. However, low-grade gliomas may also grow fast and aggressively. Since the space in the bony skull is limited and a space occupying, growing tumour may damage vital areas of the brain, any low-grade glioma can become life threatening in the course of disease. The risk of tumour cell spread (metastasis) via the cerebrospinal fluid (CSF) is generally low. Only children diagnosed with optic pathway glioma at a very young age have a slightly elevated risk of developing CSF metastases.
Incidence
With a ratio of about about 50 %, low-grade gliomas are the most common CNS tumours among children and adolescents. In Germany, about 250 children and adolescents under 18 years of age are newly diagnosed with low-grade glioma each year. This corresponds to an incidence rate of 2 to 3 per 100.000 children. Low-grade gliomas occur at all ages with a mean age at diagnosis between five and seven years. There are certain subgroups of low-grade glioma that affect younger age-groups more frequently than older children and teenagers. This is reflected by an overall highest incidence in children between two and five years of age. Boys are slightly more affected than girls (gender ratio 1.1-1.3 to 1).
Types of low-grade gliomas
The large group of low-grade gliomas comprises numerous types of tumour, which both differentiate by the way they look under the microscope (histological characteristic) as well as with regard to their biological behaviour (molecular genetic characteristics). Furthermore, glioma differ regarding their degree of malignancy, which means they show differences in how fast and aggressively they grow. The World Health Organisation (WHO) classifies low-grade gliomas either as WHO-grade I or WHO-grade II tumours (see table below). High-grade gliomas (WHO-grade III and IV tumours) are highly malignant gliomas, which are described elsewhere on this website (see information on high-grade gliomas).
WHO-grade I tumours are (with regards to their biological behaviour) benign gliomas, which usually grow slowly and show sharp margins. By growing, the tumour may push on adjacent tissue, but usually does not invade it. In contrast, WHO-grade II tumours tend to rather grow diffusely into neighbouring structures and much faster into other parts of the central nervous system. Transformation of low-grade gliomas into high-grade (highly malignant) gliomas, as has been described in adults, appears to be overall rare in children.
|
Histological type of low-grade glioma |
WHO grade |
|---|---|
|
Pilocytic astrocytoma |
I |
|
Subependymal giant cell astrocytoma |
I |
|
Other glial and mixed neuronal-glial tumours |
I |
|
Pleomorphic xanthoastrocytoma |
II |
|
Diffuse glioma |
II |
Incidences of the different (histological) subtypes of low-grade gliomas vary. Accounting for about 50-70 %, pilocytic astrocytomas (grade I) are most frequent, followed by glioneuronal tumours (grade I) with 10 % and diffuse astrocytomas (grade II) with 10 %.
Causes
Low-grade gliomas originate from malignantly transformed glial cells (neuroglia). These are cells that, among other functions, provide support and protection for the brain’s nerve cells (neurons). The exact cause for this malignant glia cell transformation is not completely defined yet.
It is known so far, that children with certain inheritated diseases (such as neurofibromatosis type 1 (NF1) or tuberous sklerosis) have a higher risk of developing low-grade glioma than their healthy peers. For example, up to 20 % of patients with NF 1 develop a low-grade glioma within the first 20 years of their life, mostly in the area of the optic pathway pathway or in the lower brain stem. Up to 15 % of patients with tuberous sclerosis are diagnosed with subependymal giant cell astrocytoma, a certain subtype of low-grade glioma, before they reach adulthood. Based on their disposition of tumour development, these kinds of genetic diseases are also known as cancer predisposition syndromes.
Also, radiotherapy of the brain in childhood, for example as received by patients with certain forms of leukaemia or with eye cancer (retinoblastoma), is associated with an increased risk of developing a CNS tumour later in life.
Symptoms
Similar to those of other tumours of the central nervous system (CNS), the presenting symptoms of low grade gliomas primarily depend on the patient’s age, tumour site and size, and pattern of spread within the CNS. The following general (nonspecific) and local (specific) symptoms can occur:
General (nonspecific) symptoms
Unspecific general symptoms occur independently of the tumour’s location. They may be similar to and therefore mimic other, non-CNS diseases. General symptoms of a child or adolescent with a CNS tumour may include headaches and/or back pain, dizziness, loss of appetite, nausea and vomiting (particularly after getting up in the morning), weight loss, increasing fatigue, inability to concentrate, school problems, mood swings, and character changes as well as developmental delay, to name a few.
Major reason for these symptoms is the slowly but continuously increasing intracranial pressure (ICP). Elevated intracranial spressure may be caused by the growing, thus more and more space-occupying tumour within the bony skull, but also by the tumour blocking the regular flow of the cerebrospinal fluid, thereby forming hydrocephalus. In babies or small children with soft spots (open fontanelles), elevated intracranial pressure and hydrocephalus typically present with a bulging fontanelle or a larger than expected head circumference (macrocephalus), respectively.
Local (specific) symptoms
Local symptoms may indicate the tumour location, thus, which functional regions of the CNS might be affected. Thus, a low grade glioma in the cerebellum can cause dizziness and gait disturbances, whereas such a tumour in the hemispheres can be associated with seizures and / or motor deficits and a tumour of the spinal cord with back pain and different kinds of motor and sensory deficits (like muscle weakness and numbness). Also, impaired vision, mental and sleep problems may, although to a lesser extent, be indicative of tumour location.
Good to know: In most children and adolescents with low-grade glioma, health problems (symptoms) develop slowly, because the tumour usually grows slowly.
Diagnosis
If the paediatrician thinks that the young patient’s history, physical exam and possibly even results from diagnostic imaging are suspicious of a tumour of the central nervous system (CNS), the child should immediately be referred to a hospital with a childhood cancer program (paediatric oncology unit), where further diagnostics can be initiated and performed by childhood cancer professionals. Very close collaboration between various specialists (such as paediatric oncologists, paediatric neurosurgeons, paediatric radiologists, to name a few) is required, both to find out whether the patient really suffers from a malignant CNS tumour and, if so, to determine the tumour type and the extension of the disease. Knowing these details is absolutely essential for optimal treatment planning and prognosis.
Tests to confirm tumour existence
The initial diagnostic procedures for a young patient presenting with a suspected CNS tumour at a childhood cancer centre include another assessment of the patient’s history, a thorough physical/neurological exam and imaging diagnostic, such as magnetic resonance imaging (MRI) and, sometimes, computed tomography (CT). These diagnostic tools help to confirm or rule out the presence of a CNS tumour and, furthermore, to assess tumour size and site and its extent with regard to the adjacent tissue. Patients with suspected optic pathway glioma additionally need a thorough eye exam performed by a specialized eye doctor. Depending on the patient’s individual situation and clinical condition, additional tests may be necessary. For example, investigation of the cerebral spinal fluid might be needed in order to identify or rule out, respectively, a spread of tumour cells into the spinal canal.
Tissue extraction (biopsy) to secure diagnosis
Final diagnosis of a CNS tumour requieres the removal of tumour tissue (biopsy). Depending on the localisation of the tumour, biopsy might be done via open surgery, for example as part of the surgery for tumour removal, or stereotactically. Stereotactic biopsy is particularly feasible to reach tumours which are located in deeper brain areas and are thus less easy to reach by conventional, open surgery. Since surgery is already part of the overall treatment concept, it should be performed by experienced paediatric neurosurgeons in a specialized treatment center. Also, the microscopic (histological) and molecular analyses of the tumour tissue are based on various specific techniques that require an experienced pathologist to obtain the correct diagnosis.
Only a few patients won't need a biopsy to confirm the diagnosis of low-grade glioma. These include patients (with and without neurofibromatosis type I), whose tumour is clearly involving the hypothalamus in the midbrain or the visual tract, respectively, and for whom an invasive procedure such as a biopsy would provide more risk than benefit. Since it is known that those lesions are most likely pilocytic astrocytomas (grade I), the interdisciplinary team of doctors may decide that in such cases tumour diagnosis is to be made by an experienced radiologist based on special features of the lesion in diagnostic imaging.
Tests before treatment begins
In preparation for the intensive treatment, further investigations may be performed, such as an X-ray to test lung function before an anaesthesia is given or electrocardiography (ECG) and echocardiograpy to check cardio function. An electroencephalogram (EEG) helps identify potential areas in the brain that the tumour might have made susceptible to cause seizures. In addition, an ophthalmological exam as well as various electrophysiological investigations to assess vision, hearing, motor and sensory functions (evoked potentials), may be required. Knowing whether the patient also has neurofibromatosis type 1 (NF I) or tuberous sclerosis, respectively, is crucial, since the concomitance of these diseases impacts the treatment strategy.
Furthermore, additional blood tests are needed to assess the patient’s general health condition and to check whether the function of certain organs (such as liver and kidneys) is affected by the disease and whether there are any metabolic disorders to be considered prior or during therapy. The condition and function of the hormonal glands will be checked in order to detect and manage potential tumour- and/or treatment-associated endocrinological impairments as early as possible. For the same reason, neuropsychological testing might be done prior to cancer treatment. Any changes occurring during the course of treatment can be assessed and managed better based on the results of those initial tests, which thus help to keep the risk of certain treatment-related side effects as low as possible.
Also, the patient’s blood group needs to be determined in case a transfusion is required during treatment. In sexually mature females (which means after they have experienced their first menstrual bleeding), a pregnancy test is recommended prior to treatment as well.
Good to know: Not every patient needs the complete check-up. On the other hand, tests might be added that haven't been mentioned here, depending on the individual situation of the patient. Your caregivers will inform you and your child, which diagnostic procedures are individually required in your case and why.
Treatment planning
After the diagnosis has been confirmed, therapy is planned. In order to design a highly individual, risk-adapted treatment regimen for the patient, certain individual factors influencing the patient’s prognosis (called risk factors or prognostic factors) are being considered during treatment planning (risk-adapted treatment strategy).
Important prognostic factors are the type, the localization, size and spread of the tumour. Also, the patient’s age and overall physical condition as well as the existence of some kind of phacomatosis (a rare syndrome such as neurofibromatosis type 1 or tuberous sklerosis) play a prognostic role. All these factors are included in treatment planning in order to achieve the best outcome possible for each patient.
Treatment
Treatment of children and adolescents with low-grade glioma should take place in a children's hospital with a paediatric oncology program. Only in such a childhood cancer centre, highly experienced and qualified staff (doctors, nurses and many more) is guaranteed, since they are specialized and focus on the diagnostics and treatment of children and teenagers with cancer according to the most advanced treatment concepts. The doctors in these centres collaborate closely with each other. Together, they treat their patients according to treatment plans (protocols) that are continuously optimised. The goal of the treatment is to achieve high cure rates while avoiding side effects as much as possible.
Treatment usually consists of surgical tumour removal (neurosurgery) followed by a controlled „watch-and-wait“-approach. By far not all patients with low-grade gliomas need chemotherapy or radiotherapy.
Surgery
For patients with low grade glioma, neurosurgical intervention aiming at removing the tumour has always been the treatment of choice. Its urgency is defined by the seriousness of the clinical symptoms and the tumour location. Since it is well-known by now that the extent of surgical tumour removal has major impact on the subsequent course of the disease, the primary goal of neurosurgery is complete tumour removal.
This strategy, however, is only applied if the benefit for the patient outweighs the risk associated with surgery, which needs to be discussed interdisciplinarily. Sometimes, however, tumours are located in parts of the brain that make complete resection impossible, because such an approach would be associated with a high risk of damaging healthy brain tissue. These patients are considered to undergo incomplete removal or a biopsy of the tumour.
Additional treatment
Children, whose tumour could be completely removed, will usually be only watched, since most of these patients do not experience recurrent disease.. If a complete removal of the tumour is not possible, the doctors will discuss whether the patient should receive non-surgical treatment, such as chemo- and radiotherapy, or not. If not, the patient will be observed by regular clinical and imaging check-ups (controlled „watch-and wait“-approach).
The experts agree that non-surgical therapy at the time of diagnosis should only be recommended for patients presenting with severe clinical symptoms (such as diencephalic syndrome or rapid loss of visual acuity). For even after only incomplete tumour removal, many children and teenagers neither show signs of tumour growth nor any other severe health problems and their probability of survival is high. Therefore, the recommendation for these patients is, like for children after complete removal, the controlled watch-and-wait approach rather than chemo- or radiotherapy.
In case of any signs of tumour growth or if the patient presents with deteriorating clinical symptoms during one of the check-ups, the pros and cons of a second neurosurgical intervention will be evaluated. Alternatively, if second surgery is not considered beneficial or feasible, either chemo- or radiotherapy are an option. The doctors will carefully evaluate the optimal timepoint and strategy for non-surgical treatment.
Until recently, the patient's age was the primary factor with regards to chose between chemo- and radiotherapy. Chemotherapy was usually chosen for treating younger children, for whom radiation would not be an option. Today, however, it is well known that chemotherapy will not reduce the efficacy of subsequent radiotherapy. Therefore, experts from many different countries have agreed on chemotherapy as the non-surgical treatment of choice.
In case of progressive or recurrent disease, chemotherapy may be repeated, even multiple times and with different agents. The goal is to postpone radiotherapy as long as possible, because especially younger children's developing brains are highly sensitive to radiation-induced damage and subsequent long-term side effects. Only if chemotherapy remains unsuccessful, radiation is being considered an alternative, except for patients younger than one year or with metastasized disease.
Chemotherapy
Chemotherapy uses drugs (so-called cytostatic agents) that can kill fast-dividing cells, such as cancer cells, or inhibit their growth, respectively. Treatment involves multiple agents to provide highest possible efficacy in eliminating tumour cells. Standard European treatment regimes include vincristine and carboplatin. Additionally or alternatively to these agents (for example in case of adverse effects or non-response), other drugs may be given as well (such as etoposide, cyclophosphamide or cisplatin).
As per current protocols, treatment of patients with low-grade gliomas consists of two major parts: induction and consolidation phase, each of which include several treatment blocks. The first (intensive) phase serves to shrink the remaining tumour or to stop it from growing, respectively, the second phase is supposed to maintain or even improve the previous treatment results.
Radiotherapy
Radiotherapy is done using energy-rich, electromagnetic radiation, given through the skin to the tumour region. Radiation causes DNA damage in tumour cells, thereby leading to cell death. Patients with low-grade gliomas usually receive a total radiation dose between 50 and 54 Gray (Gy) over a period of five to six weeks. Modern radiation techniques, such as Intensity-Modulated Radiotherapy (IMRT) help minimise the damage of healthy tissue. For some patients, radiotherapy with protons instead of conventional radiotherapy (with photons) may be an option, for example for very young children or in case proton therapy is expected to have a clear advantage compared to conventional radiotherapy.
Special therapeutic situations – patients with neurofibromatosis or tuberous sclerosis
Aside from their disposition of developing multiple tumours, patients with neurofibromatosis type I (NF I) may also present with NF-associated cognitive impairment. In order to prevent radiotherapy-induced brain damage, chemotherapy (for example with carboplatin and vincristine) is given instead of radiotherapy. Research has shown that in NF I-patients chemotherapy is very efficient.
Therapy optimising trials and registries
In the large paediatric treatment centres, children and teenagers with low-grade glioma receive therapy according to standardised protocols. These protocols are designed by experts and provide consistent concepts for chemo- and radiotherapy based on the patient’s age at diagnosis as well as concomitant diseases such as NF1. The goal is to stop the tumour from growing while also reducing the risk of disease-related late effects (such as vision impairment by optic pathway glioma). Therefore, children and teenagers with low-grade glioma usually receive therapy according to the treatment plans of therapy optimising trials or registries.
Therapy optimising trials are standardised and controlled clinical trials that aim at steadily developing and improving treatment concepts for sick patients based on the current scientific knowledge. Patients who cannot participate in any study, for example because none is available or open for them at that time, or since they do not meet the required inclusion criteria, respectively, are often included in a so-called registry. The registry center supports the doctors at site with (non-commital) treatment recommendations based on the most recent data on best treatment options, in order to provide the patient with optimal therapy even without the framework of a clinical study.
Current situation in Germany
Currently, there are no therapy optimising trials available for children and adolescents with low-grade glioma in Germany. The last trial (SIOP-LGG 2004) was closed for patient registration early 2012; a subsequent trial has not yet been opened, but is being planned.
Between April 2012 and the end of 2018, children and adolescents with low-grade glioma were enrolled in the Registry SIOP-LGG 2004. The registry’s headquarters at the Children’s Cancer Centre of Augsburg (supervised by Dr. med. A. Gnekow) provided therapy recommendations based on the results of approved diagnostic and treatment standards of trial SIOP-LGG 2004 (which had been closed in 2012). Since the beginning of 2019, the registry SIOP-LGG 2004 has been closed for patient registration. Patients that had been enrolled in the registry by the end of 2018, however, were further attended to by the SIOP-LGG study centre (Augsburg) until recently.
Important: As from 01/09/2020, there will be no more guidance by the SIOP-LGG study centre. All patients, who have been cared for as per SIOP-LGG Registry so far, are asked to get involved with the LOGGIC Registry if needed (see below).
Currently, the following international registriy is available for patients with low-grade glioma in Germany:
- LOGGIC Registry: Since 01/01/2019, the LOGGIC Registry recruits children and adolescents under 21 years of age in Europe, who have been newly diagnosed with low-grade glioma (LOGGIC stands for Low Grade Glioma In Children). Additionally, the LOGGIC Core BioClinical Data Bank was opened in April 2019 for documentation of molecular tumour characteristics. LOGGIC Registry and LOGGIC Core aim at comprehensive (including molecular) data collection from patients with LGG. Goal is to improve the understanding of the biology of these tumours as a basis for novel treatment designs. The LOGGIC Registry headquarters are located at the Department of Paediatric Oncology and Haematology at the Charité in Berlin (principal investigator: PD Dr. med. P. Hernáiz Driever). The LOGGIC Core headquarters are located at the Hopp Children’s Cancer Center Heidelberg (Hopp-Kindertumorzentrum, KiTZ; principal investigator: Professor Dr. med. O. Witt).
Prognosis
The prognosis for children and adolescents with low-grad glioma has significantly improved due to the standardized treatment concepts. In addition, modern diagnostic and surgical methods have contributed to improve outcome. The chance of survival is very good for most children and adolescents with a low-grade glioma: More than 90 % of all patients survive their disease longterm, however, many of them with remaining tumour and/or disease- or treatment-related late effects.
Treatment options and, thus, prognosis are (also) markedly dependent on the tumour site and the possibilities of neurosurgical intervention. Hence, patients with low-grade astrocytomas of the cerebrum and cerebellum achieve long-term survival rates of 100 % after complete tumour removal, while prognosis is less favourable in patients after inclomplete resection or with tumours in other regions of the brain.
Also, potential tumour- and/or treatment related neurological, ophthalmological, endocrinological, intellectual, and psychosocial deficits can negatively affect life quality of patients with low grade glioma. Future therapy strategies are, therefore, both aiming at improving patients‘ survival and reducing late-effects of tumour and therapy on conditions such as neurology, endocrinology, ophthalmology and intellectual development. The assessment of how the treatment impacts the survivors’ health and quality of life is major part of current and future studies.
References 
- Kaatsch P, Grabow D, Spix C: German Childhood Cancer Registry - Anual Report 2018 (1980-2017). Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz 2019 [URI: www.kinderkrebsregister.de]
- Fleischhack G, Rutkowski S, Pfister SM, Pietsch T, Tippelt S, Warmuth-Metz M, Bison B, van Velthoven-Wurster V, Messing-Jünger M, Kortmann RD, Timmermann B, Slavc I, Witt O, Gnekow A, Hernáiz Driever P, Kramm C, Benesch M, Frühwald MC, Hasselblatt M, Müller HL, Sörensen N, Kordes U, Calaminus G: ZNS-Tumoren. in: Niemeyer C, Eggert A (Hrsg.): Pädiatrische Hämatologie und Onkologie. Springer-Verlag GmbH Deutschland, 2. vollständig überarbeitete Auflage 2018, 359 [ISBN: 978-3-662-43685-1]
- Gnekow AK: Gliome niedrigen Malignitätsgrades im Kindes- und Jugendalter. Leitlinie der Gesellschaft für Pädiatrische Onkologie und Hämatologie 2018 [URI: www.awmf.org]
- Ripperger T, Bielack SS, Borkhardt A, Brecht IB, Burkhardt B, Calaminus G, Debatin KM, Deubzer H, Dirksen U, Eckert C, Eggert A, Erlacher M, Fleischhack G, Frühwald MC, Gnekow A, Goehring G, Graf N, Hanenberg H, Hauer J, Hero B, Hettmer S, von Hoff K, Horstmann M, Hoyer J, Illig T, Kaatsch P, Kappler R, Kerl K, Klingebiel T, Kontny U, Kordes U, Körholz D, Koscielniak E, Kramm CM, Kuhlen M, Kulozik AE, Lamottke B, Leuschner I, Lohmann DR, Meinhardt A, Metzler M, Meyer LH, Moser O, Nathrath M, Niemeyer CM, Nustede R, Pajtler KW, Paret C, Rasche M, Reinhardt D, Rieß O, Russo A, Rutkowski S, Schlegelberger B, Schneider D, Schneppenheim R, Schrappe M, Schroeder C, von Schweinitz D, Simon T, Sparber-Sauer M, Spix C, Stanulla M, Steinemann D, Strahm B, Temming P, Thomay K, von Bueren AO, Vorwerk P, Witt O, Wlodarski M, Wössmann W, Zenker M, Zimmermann S, Pfister SM, Kratz CP: Childhood cancer predisposition syndromes-A concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. American journal of medical genetics. Part A 2017, 173: 1017 [PMID: 28168833]
- Gnekow AK, Walker DA, Kandels D, Picton S, Giorgio Perilongo, Grill J, Stokland T, Sandstrom PE, Warmuth-Metz M, Pietsch T, Giangaspero F, Schmidt R, Faldum A, Kilmartin D, De Paoli A, De Salvo GL, of the Low Grade Glioma Consortium and the participating centers: A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma - A final report. European journal of cancer (Oxford, England : 1990) 2017, 81: 206 [PMID: 28649001]
- Rutkowski S, Trollmann R, Korinthenberg R, Warmuth-Metz M, Weckesser M, Krauss J, Pietsch T: Leitsymptome und Diagnostik der ZNS-Tumoren im Kindes- und Jugendalter. Gemeinsame Leitlinie der Gesellschaft für Neuropädiatrie und der Gesellschaft für Pädiatrische Onkologie und Hämatologie 2016 [URI: www.awmf.org]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica 2016, 131: 803 [PMID: 27157931]
- Gnekow AK, Falkenstein F, von Hornstein S, Zwiener I, Berkefeld S, Bison B, Warmuth-Metz M, Driever PH, Soerensen N, Kortmann RD, Pietsch T, Faldum A: Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro-oncology 2012, 14: 1265 [PMID: 22942186]
- Hernáiz Driever P, von Hornstein S, Pietsch T, Kortmann R, Warmuth-Metz M, Emser A, Gnekow AK: Natural history and management of low-grade glioma in NF-1 children. J Neurooncol 2010, [PMID: 20352473]
- Calaminus G: Lebensqualität bei Kindern und Jugendlichen mit Hirntumoren. WIR Informationsschrift der Aktion für krebskranke Kinder e.V. (Bonn) 2004, 2: 6 [URI: www.kinderkrebsstiftung.de]
- Gnekow AK: Therapie von Gliomen niedriger Malignität im Kindes- und Jugendalter. WIR Informationsschrift der Aktion für krebskranke Kinder e.V. (Bonn) 2003, 2: 8 [URI: www.kinderkrebsstiftung.de]
- Listernick R, Louis DN, Packer RJ, Gutmann DH: Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol 1997, 41: 143 [PMID: 9029062]


 PDF Brief Information on Low-Grade Glioma (428KB)
PDF Brief Information on Low-Grade Glioma (428KB)